top of page
Plasmas
We will first take a look at what plasmas are.
 A "basic" plasma has a mixture of ions, radicals, electrons and neutral species in a gaseous mass, which is confined and at a reduced pressure. |  Formation of a plasma through electron collisions. |  A plasma needs electrons to dissociate gas molecules into radicals and ions and also to maintain the source of electrons. Energy must be continually added to sustain the plasma by giving free electrons sufficient momentum with which to collide and break bonds and to generate more free electrons. |
|---|---|---|
 |  |  |
 |  |  |
 |  It is important to keep in mind that there are always several reactions occurring. |  A plasma is also continiously radiating light as atoms relax back into the ground state through electron transitions. This gives a useful diagnostic. |
 Once a plasma is initiated, RF power must be continually supplied to it. Gas flow, chamber pressure and gas species can be varied however. Changes will depend on the application. |  |  Typical Maxwell-Boltzmann electron-energy distribution. There aren't usually many electrons available to form ions. RF energy has to be sufficient to populate the electron density at higher energies. This is a two-fold process since RF power gives more electrons and more energetic electrons. |
 |  |  |
 |  |  |
 Residence time is a very important parameter for both profile and etch rate. |  |  |
 |  |  |
 After being able to generate and sustain a stable plasma, choice of suitable gases allows chemical etching. F and/or Cl radicals are found to etch most materials. Both F and Cl form volatile by-products which go into the plasma before leaving the chamber as gases are continiously replenished in a flushing manor. |  Etching arises from chemically reactive species in the plasma arriving at the sample surface and undergoing a chemical reaction. F and Cl radicals are very reactive and most etching plasma have either F or Cl present. RIE etching requires ions too, these give bombardment which helps break bonds, helps to form protective passivation layers and can sputter materials. Larger atoms like Ar and Xe are good at forming +ve ions since their outermost electrons are loosely bound. |  It is essential that the etching by-products are volatile and can be pumped away. Otherwise they would remain on the wafer and act as a mask, leading to major problems. |
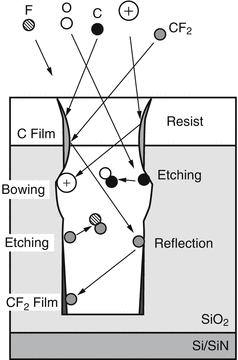 Schematic of the overall process. Chemical etch from reactive radicals, passivation forming by action of suitable polymer forming species. Ion bombardment and scattering which must be controlled by pressure and voltage to get optimum performance. |
bottom of page